If you rub two materials together, sometimes the materials get opposite charges. This effect is longer lasting in dry weather because the water in the air allows the charges to return to equilibrium.
Experiment: Try sticking two strips of scotch tape together and quickly pealing them apart. The strips should now be oppositely charged. As you bring them near each other, they should be attracted.
Try repeating the experiment again to make 4 strips of tape. Put the newly charged tape near the old and some will repel instead of attract.
Question: After playing with the tape what factors determine the strength of the force?answer
The force between the tape strips is strong when they are closer.
The force also varies with how much charges is separated. Separating more charge is easier when the humidity is lower.
Some particles have an electric charge. The rules that govern these charges can explain electricity, chemical bonds, magnetism, and light!
Electric charge determines the magnitude and direction of the electrostatic force. Charge has a role in the electrostatic force similar to mass's role in gravity.
opposite charges have an attractive force
negative charges have a repulsive force
positive charges have a repulsive force
neutral charges have no force
Charge can be neutralized. If a positive and negative charge are close together the attractive and repulsive forces mostly cancel each other out. This explains why we don't notice the electrostatic forces from neutral atoms.
Electric charge can't be created or destroyed. Like energy and momentum, electric charge is conserved.
The evidence for this conservation law is based on repeated experiments. No one has ever documented the total charge of a system increasing or decreasing. This law is important in particle physics where charged particles can be created and destroyed, but only when another particle is created or destroyed to balance out the total charge.
Elementary Electric Charge
In 1909 Robert Millikan and Harvey Fletcher performed the oil drop experiment to investigate electric charge. They sprayed a fine mist of oil into a uniform electric field. The electric field produced a force on some of the oil droplets. Based on that force, they found that charge only came in even multiples of about 1.6 × 10−19 C.
This evidence helped build our modern model of the atom: negative electrons bound to a nucleus of positive protons and neutral neutrons. Most charge comes from electrons or protons, but there are also more exotic particles.
charge = −1.602 × 10−19 C
mass = 9.109 × 10−31 kg
charge = +1.602 × 10−19 C
mass = 1.672 × 10−27 kg
solution
Charge has only been observed in packets of 1.602 × 10−19 C. Any recorded charge must be a multiple of this value.
Conductivity
Conductive materials allow electric charges to easily move through them. If a charge is applied to one part of a conductive material the charge will quickly spread out because like charges repel.
These simulations don't include the nuances of quantum mechanics, they only show a cartoony approximation of conductivity.
In chemistry, elements are roughly divided into metals, metalloids and nonmetals. Metals are held together by loosely sharing their outer valence electrons. The cloud of free flowing electrons give metals most of their shared characteristics, like conductivity.
insulators
vacuum, nonmetals: gases, plastics, silk, fur
semi-conductors
metalloids: carbon and silicon
electrolytes
solvents with dissolved ions:
salt water, tap water, soda water
conductors
metals, plasma
superconductors
certain low temperature ceramics
air, coca cola, copper, carbon, plastic fork
answer
high conductivity
copper (conductor)
coca cola (electrolyte)
carbon (semi-conductor)
plastic fork (insulator)
air (insulator)
low conductivity
answer
In metals some electrons are free to move between atoms. In nonmetals the electrons are locked up in covalent bonds so they resist the electrostatic force.
Another way to make a substance conductive is to heat it up so much that electrons can leave the nucleus. We call this state of matter a plasma.
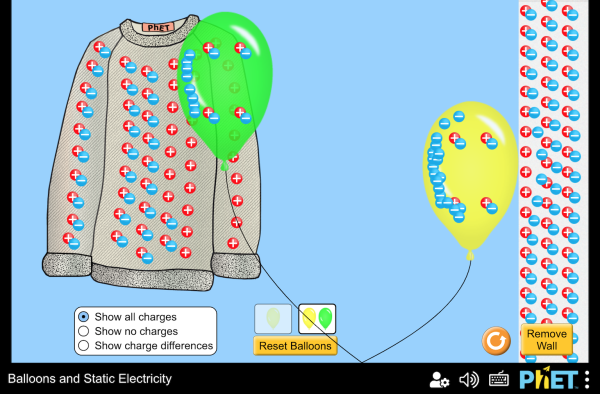
Static Electricity
It's easy to separate a couple trillion electrons from their protons by walking with socks on a carpet. Lightning is produced in a similar way when a cloud with rising air ends up with an unbalanced distribution of charge.
Static electricity occurs when there is an imbalance of electrons and protons. A lasting charge separation can only occur in insulating materials, because in conductors positive and negative charges quickly pair up.
Static electricity effects are much stronger and longer lasting in low humidity. This is because water molecules increase the conductivity of air, allowing more separated charges to return.
Play around with this PhET simulation for static electricity.
Question: Which are conductors and which are insulators:
(balloons, sweater, wall, air)
answer
conductors: nothing in this simulation
insulators: balloon, sweater, wall, air
Why not the other way around?
answer
Some materials are better at holding onto extra electrons for complex quantum mechanical reasons.
answer
After rubbing, the balloon has unpaired negative charge, and the sweater has unpaired positive charge. Opposite charges attract.
answer
After rubbing, the balloon has unpaired negative charge. When charge is near another insulator it repels the electrons enough that they are slightly farther away, but not enough to cause them to leave the nucleus. This charge separation is called polarization.
The polarization of the positive and negative pairs creates an induced charge. The charged balloon causes the wall to polarize, which increases the attraction and decreases the repulsion between the balloon and wall.
A TriboElectric Series lists which materials will become electrically charged after they are rubbed together.
Question: If you rubbed polystyrene foam (styrofoam) on a cat, static electricity would cause them stick together. Which would gain a positive charge?answer
The cat would end up with a positive charge. This means the cat would lose electrons to the styrofoam.
answer
The glass would get the positive charge.